Carbonyl Polymerization
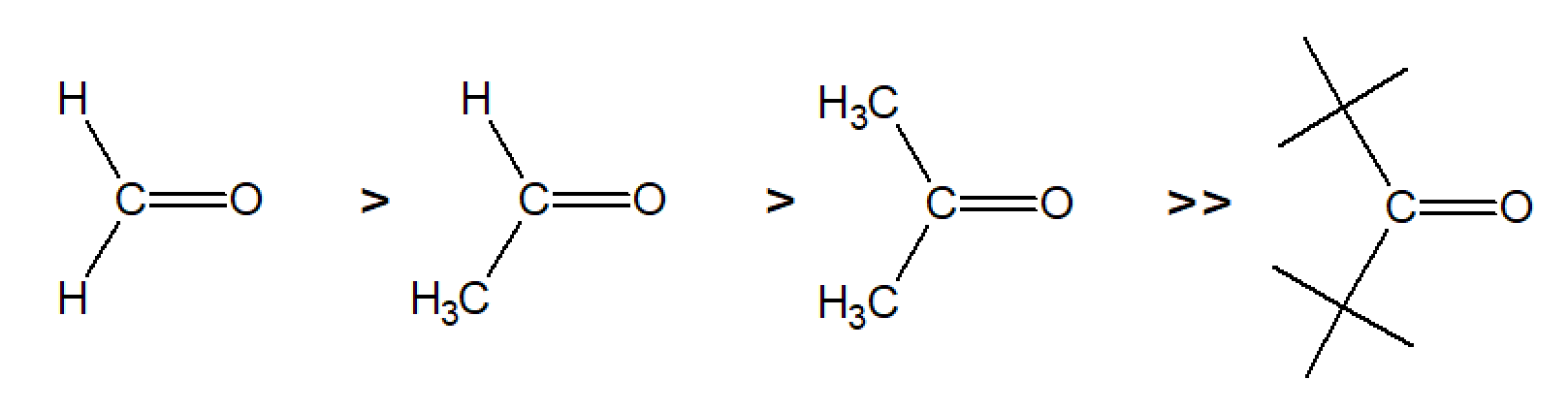
Chemical active carbonyl groups, C=O, can be found in many organic compounds including aldehydes, dicarboxylic acids, acyl halides, diketones and keto esters. The carbonyl bond is not only strong but also very reactive since it is highly polarized due to the large difference of the electronegativities of the carbon and oxygen atoms. One of the most reactive carbonyl compounds is formaldehyde (methanal) due to its relative weak bond of about 180 kcal/mol and small size of the hydrogen substituents whereas carbon monoxide has the strongest bond (257 kcal) and thus is the least reactive carbonyl compound.1, 7-8 The reactivity also depends on the size of the substituents i.e. their bulkiness. The reactivity decreases in the order
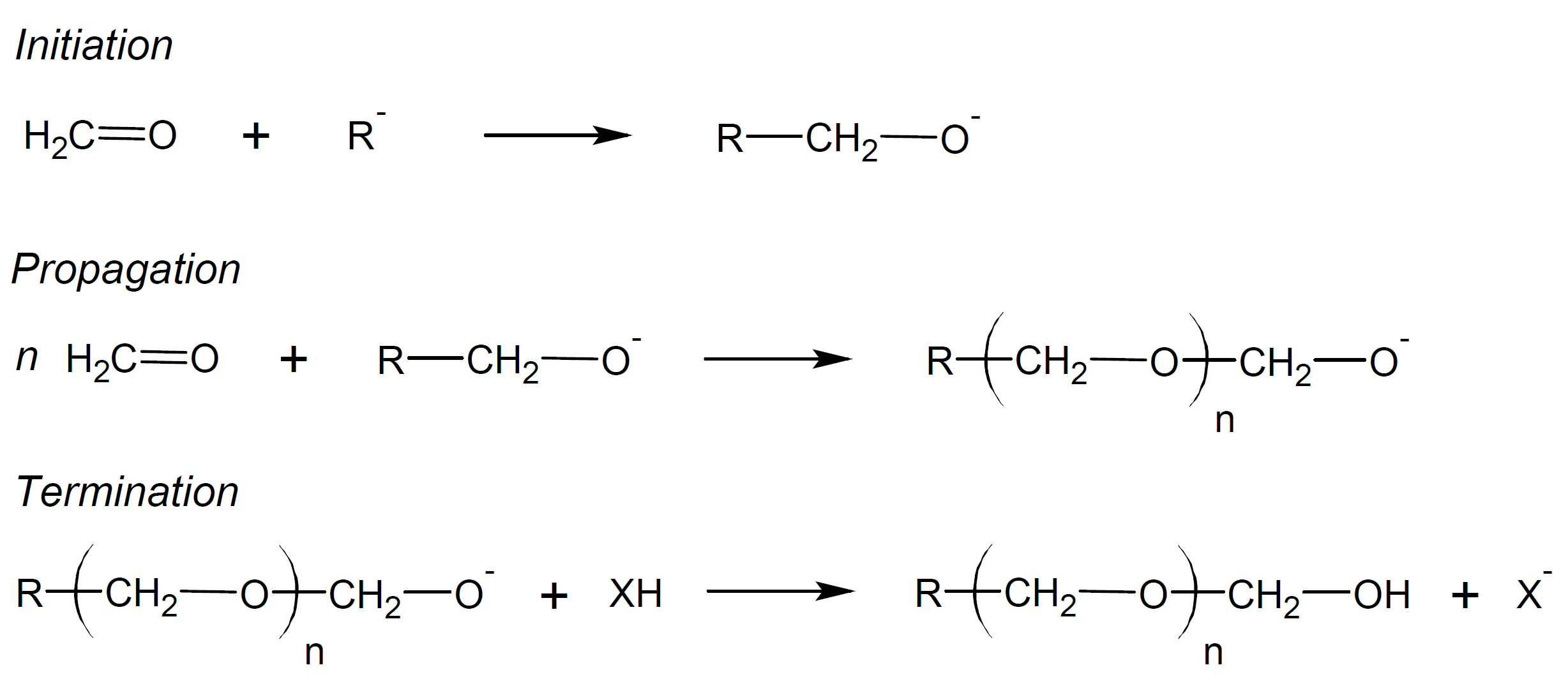
Like carbon double bonds, the C=O bond can undergo polymerization reactions. However, unlike olefins, carbonyl monomers are not very susceptible to polymerization by radical initiators due to the instability of the carbonyl radical but are prone to nucleophilic and electrophilic attack, and thus can undergo anionic and cationic polymerization. One of the most prominent examples is anionic polymerization of formaldehyde to polyacetal (also called polyoxymethylene or polyformaldehyde). Anionic chain growth can be initiated by a large number of compounds including metal alkyls, metal alkoxides, amines, phosphines and some other organic compounds.2,3
The polymerization proceeds in three steps:
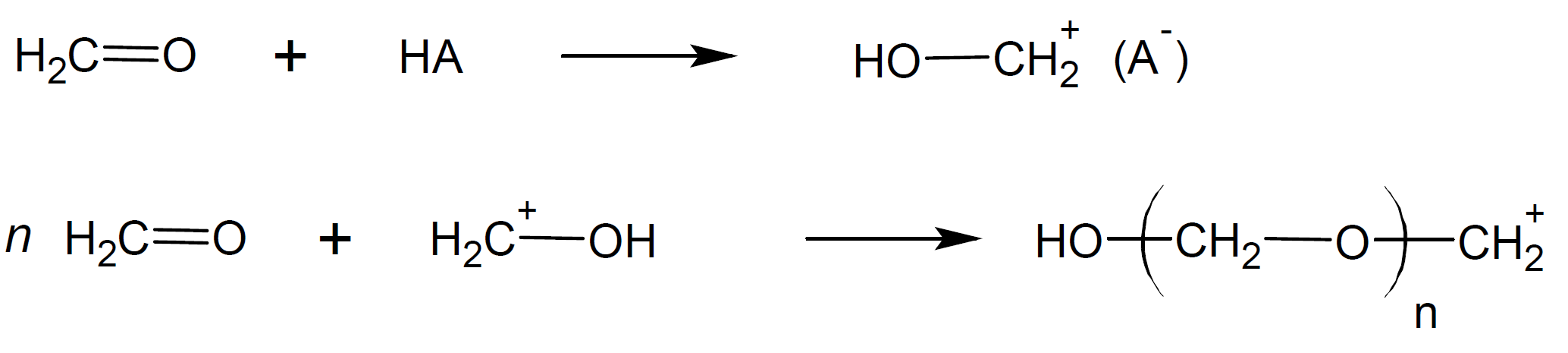
Most commercial polyacetals are produced by anionic polymerization. However, formaldehyde and many other carbonyl compounds can also be readily produced by cationic polymerization. The mechanism is very similar to that for anionic polymerization, however, high molecular weight polymers can only be prepared with strong cationic initiators that form very stable cations because cationic growth centers readily undergo chain termination by recombination with anion fragments.3 Some effective initiators include strong protonic acids such as hydrochloric acid and Lewis acids of metal halides.
Some other monomers that (readily) undergo ionic chain polymerization include acetaldehyde and higher aliphatic aldehydes4, haloaldehydes (chloral, fluoral, bromal), (methyl)glyoxal as well as dialdehydes with 2-3 carbon atom spacing such as glutaraldehyde, succinaldehyde, and phthalaldehyde. Glyoxal yields highly crosslinked polymers whereas most other dialdehydes cyclopolymerize in the presence of acid initiators into relatively low molecular weight polymers with five- and six-membered rings.2
Most aldehydes and ketones have a low ceiling temperature (Tc) which is either at or noticeably below ambient temperature which means that these compounds do not polymerize at or above room temperature. The low Tc values are due to the low molar free enthalpy of polymerization of carbonyl double bonds which is appreciably lower than that of vinyl monomers whereas their entropy of polymerization is similar. The low Tc values explain why most of the early efforts to polymerize carbonyl compounds were unsuccessful since they were carried out at too high temperatures (T > Tc). Some exceptions are polyformaldehyde (Tc = 120°C) and certain halogenated polyaldehydes such as polytrifluoroacetaldehyde (Tc = 85°C) whereas most other aldehydes have Tc values well below room temperature.2 To prevent these polymers to depolymerize, they have to be end capped with more stable groups, for example by converting the less stable hydroxyl end groups to carboxyl groups which has shown to effectively prevent unzipping of the polymer chain.5,6
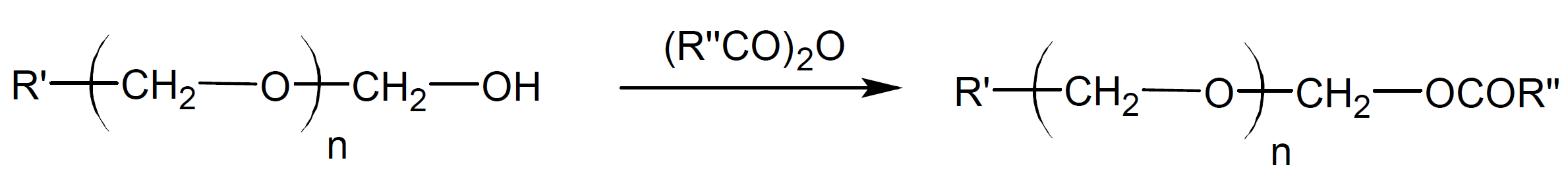
This reaction is known as end capping or end blocking.
References and Notes
- G. Glockler, J. Phys. Chem., 62, 9, 1049-1054 (1958)
- O. Vogl, J. Poly. Sci.: Part A: Poly. Chem., Vol. 38, 2293-2299 (2000)
- P. Kubisa, I. Negulescu, K. Hatada, D. Lipp, J. Starr, B. Yamada and 0. Vogl, Pure & Appl. Chem., Vol., 48, pp. 275-285 (1976)
- Formaldehyde, acetaldehyde, and higher aliphatic aldehydes may also form cyclic trimers and tetramers. The cyclic trimers of higher aldehydes, however, cannot be polymerized by ring opening polymerization because the ceiling temperature for cyclomerization is typically higher than that for polymerization.2
- A common capping agent is acetic anhydride.6
- US Patent 3,687,898; T. Ishii, S. Sugiura and N. Takikawa; Process for the production of heat-resistant oxymethylene polymer (1972)
- S.J. Blanksby, G.B. Ellison, Acc. Chem. Res., 36, 4, 255-263 (2003)
- The bond energy of carbonyl groups decreases in the order CO > CO2 > ketenes > aldehydes, ketones ≈ carboxylic acids, esters, whereas the single C-O bonds of alcohols and ethers have much lower bond energies (< 100 kcal/mol).7